Rapid Check™
RSV Antigen Test
CLIA Waived testing at the Point-of-Care for use
Rapid, qualitative test for the detection of Respiratory Syncytial Virus (RSV)
Features and Benefits:
- Rapid Detection and Patient Management
RSV Infection results available in 15 minutes
- Various Sample Types
Nasopharyngeal Swab and Nasal Aspirates from symptomatic patients less than 6 years and over the age of 60.
- Simple and Effective
CLIA Waived testing at the point-of-care for use with minimally trained healthcare professionals
- Premier Test Performance*
Positive Percent Agreement: 93. 9%
Negative Percent Agreement: 97.7%
*Nasopharyngeal Swab and Nasal Aspirates. Refer to the Package Insert for additional performance claims.
- Transport Medium Compatibility
Allows for analysis of preserved or transported specimens.
- External Quality Controls Included
Facilitate routine quality inspection
- Shelf Life that Spans Viral Seasonality
24 months from date of manufacture at room temperature storage
Kit Contents:
- 20 Individually packaged test devices
- 20 Extraction Tubes
- 40 Disposable Pipettes
- 1 PC (Positive Control Reagent)
- 1 NC (Negative Control Reagent)
- 1 R1 (Extraction Reagent Solution)
- 1 Instructions for Use
Principle
The Rapid Check™ RSV Antigen test is a lateral flow immunogold assay for the direct visual detection of RSV
protein F in clinical samples. The basis for protein F detection is in the use of a red - colored gold labeled
mouse monoclonal anti-RSV protein F antibody that after addition of extracted sample travels laterally along
the strip test device membrane. This lateral flow carries the mixture of sample and gold labeled anti-RSV
protein F through a membrane adsorbed monoclonal anti-RSV protein F Test Line (T) and then through a
membrane adsorbed goat an anti-mouse immunoglobulin Control Line (C). When RSV protein F is present in
clinical samples, the fluid phase mouse anti-RSV protein F binds this antigen and this formation of antigen -
antibody complex is then in turn bound at the Test Line (T). The unbound or excess mouse anti-RSV protein
F passes through the Test Line (T) and is bound at the Control Line (C) by goat anti- mouse immunoglobulin.
Therefore, in the presence of RSV protein F antigen, 2 red lines become visible: one at the Test Line (T) and
a second at the Control Line (C). But when RSV antigen is absent only one red line appears at the Control
Line (C).
Test Procedure
|
|
|
|
|
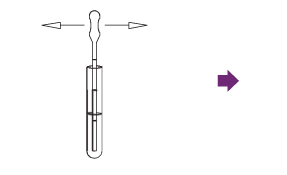
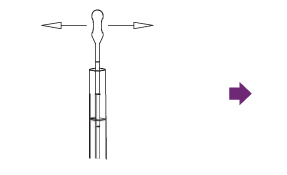
Draw 160 µL (second notch) of the sample up into a disposable pipette.
|
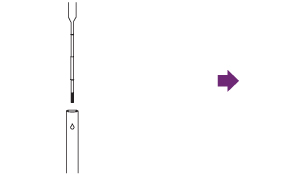
Add entire contents of the Pipette to the extraction tube.
|
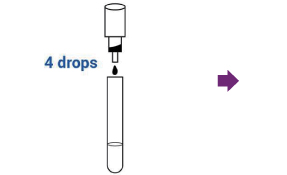
Add four (4) free falling drops of R1 to the extraction tube while holding the bottle vertically over the tube.
|
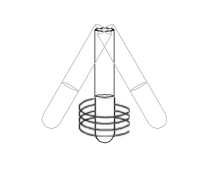
Gently swirl the extraction tube to mix the sample and R1.
|
|
|
|
|
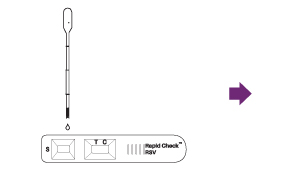
Draw 160 µL (second notch) of the sample up into a disposable pipette.
|
Squeeze entire contents of the pipette dropper onto the sample well of the test device.
|
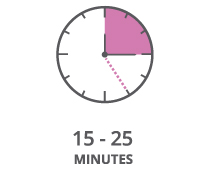
Read test result between fifteen (15) and twentyfive (25) minutes after applying the extracted sample to the test device.
|
Results read outside the recommended time range are considered invalid.
Results Interpretations
The test result should be read between fifteen (15) and twenty-five (25) minutes after applying the extracted
sample to the test device.
If the results are read after 25 minutes, the result is invalid. Specimen should be retested.
Positive Result
For a positive specimen, the appearance of TWO pink to red lines, one at the procedural control line (C) and
one at the test line (T) indicates the presence of respiratory syncytial virus antigen. Any pink to red test line,
even if it is only slightly pink, is considered a positive test.
Results reported: Positive for the presence of RSV antigen. A positive result may occur in the presence of
both viable and non-viable viruses.
Negative Result
For a negative specimen, the appearance of ONLY ONE pink to red line at the procedural control line (C) and
no pink to red line at the Test Line (T) indicates that the sample is negative for RSV viral antigen.
Results reported: Negative for presence of RSV antigen. Infection due to RSV cannot be ruled out since the
antigen present in the sample may be below the detection limit of the test. Cell culture confirmation of
negative samples is recommended.
Invalid Result
If after 15 minutes, the pink to red procedural control line (C) does not appear, even if any shade of pink to
red test line (T) appears, the result is considered invalid. If the test is considered invalid because a control
line fails to appear, the test should be repeated with a new test device.
Within minutes the result area should be white to light pink and allow clear interpretation of test result.
If the background color persists and interferes with the interpretation of the test result, the result is
considered invalid
.
Should this occur, review the test procedure and repeat the test with a new test device.
If the test result is still invalid after repeating with a new test device, then it is considered invalid.
Warnings and Precautions
- The Rapid Check™ RSV Antigen Test is for in vitro diagnostic use.
- The positive control (PC) is made with Clorox inactivated RSV and should be handled as though it could
transmit disease.
- Do not use the kit contents beyond the expiration date printed on the outside of the box and on the
individual components.
- Use appropriate precautions against microbial hazards in the collection, handling, storage, and disposal
of patient samples and used kit contents.2 Discard used material in a proper biohazard or sharps
container. Patient samples should be handled as though they could transmit disease.
- The test device must remain sealed in the protective foil pouch until use.
- The R1, PC, and NC contain a detergent solution. If the solution contacts the skin or eye, flush with
copious amounts of water.
- To obtain accurate results, you must follow the test procedure in the package insert.
- To obtain accurate results, use appropriate nasopharyngeal swabs and recommended transport media.
- All transfer pipettes and test vials are single-use items. do not use more than once.
