Fecal Occult Blood Rapid Test Cassette (Feces)
Benefits of a CLIA Waived Fecal Occult Blood Test - iFOBT
When the MD CLIA Waived Fecal Occult Blood Test is performed, it designed to detect the presence of blood in stool specimens. The presence of of human hemoglobin is detected with special antibodies makes MD iFOBT test a more accurate testing tool than a standard FOBT test, to ensure effective screening for colorectal cancer.
INTENDED USE
Fecal Occult Blood Rapid Test Cassette (Feces) is a rapid chromatographic immunoassay for the qualitative detection of human occult blood in feces by professional laboratories or physician's offices. It is useful to detect bleeding caused by a number of gastrointestinal disorders, e.g., diverticulitis, colitis, polyps, and colorectal cancer.
Fecal Occult Blood Rapid Test Cassette (Feces) is recommended for use in
1) routine physical examinations, 2) hospital monitoring for bleeding in patients, and 3) screening for colorectal cancer or gastrointestinal bleeding from any source.
INTRODUCTION
Most of diseases can cause hidden blood in the stool. In the early stages, gastrointestinal problems such as colon cancer, ulcers, polyps, colitis, diverticulitis, and fissures may not show any visible symptoms, only occult blood. Traditional guaiac-based method lacks sensitivity and specificity, and has diet-restriction prior to the testing.
Fecal Occult Blood Rapid Test Cassette (Feces) is a rapid test to qualitatively detect low levels of fecal occult blood in feces. The test uses double antibody-sandwich assay to selectively detect as low as 100ng/ml of hemoglobin or 12µg hemoglobin/g feces. In addition, unlike the guaiac assays, the accuracy of the test is not affected by the diet of the patients.
PRINCIPLE
Fecal Occult Blood Rapid Test Cassette (Feces) is a lateral flow chromatographic immunoassay based on the principle of the double antibody-sandwich technique. The membrane is pre-coated with anti-hemoglobin antibodies on the test line region of the device. During testing, the specimen reacts with the colloidal gold coated with anti-hemoglobin antibodies. The mixture migrates upward on the membrane chromatographically by capillary action to react with anti-hemoglobin antibodies on the membrane and generate a colored line. The presence of this colored line in the test region indicates a positive result, while its absence indicates a negative result. To serve as a procedural control, a colored line will always appear in the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
STORAGE AND STABILITY
All reagents are ready to use as supplied. Store unused test device unopened at 2°C-30°C. If stored at 2°C-8°C, ensure that the test device is brought to room temperature before opening. The test is not stable out of the expiration date printed on the sealed pouch. Do not freeze the kit or expose the kit over 30°C.
PRECAUTIONS
- For professional In Vitro diagnostic use only.
- This package insert must be read completely before performing the test. Failure to follow the insert gives inaccurate test results.
- Do not use it if the tube/pouch is damaged or broken.
- Test is for single use only. Do not reuse under any circumstances.
- Do not use specimen with visible blood for the testing.
- Handel all specimens as if they contain infectious agents. Observe established standard procedure for proper disposal of specimens.
- Specimen extraction buffer contains Sodium Azide (0.1 %). Avoid contact with skin or eyes. Do not ingest.
- Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are assay.
- Humidity and temperature can adversely affect results.
- Do not perform the test in a room with strong air flow, ie. electric fan or strong air conditioning.
PATIENT PREPARATION
- A specimen should not be collected from a patient with following conditions that may interfere with the test results:
- Menstrual bleeding
- Bleeding hemorrhoids
- Constipating bleeding
- Urinary bleeding
- Dietary restrictions are not necessary.
- Alcohol and certain medications such as aspirin, indomethacin, phenylbutazone, reserpine, corticosteroids, and non-steroidal anti-inflammatory drugs may cause gastrointestinal irritation and subsequent bleeding, thus gives positive reactions. On the advice of the physician, such substances should be discontinued at least 48 hours prior to testing.
SPECIMEN COLLECTION AND PREPARATION
Consider any materials of human origin as infectious and handle them using standard biosafety procedures.
- Collect a random sample of feces in a clean, dry receptacle.
- Unscrew the top of the collection tube and remove the applicator stick.
- Randomly pierce the fecal specimen in at least five (5) different sites.
- Remove excess sample off the shaft and outer grooves. Be sure sample remains on inside grooves.
- Replace the stick in the tube and tighten securely.
- Shake the specimen collection bottle so that there is proper homogenization of feces in buffer solution.
Note:
Specimens prepared in the specimen collection tube may be stored at room temperature (15-30°C) for 3 days maximum, at 2-8°C for 7 days maximum or at -20°C for 3 months maximum if not tested within 1 hour after preparation.
TEST PROCEDURE
Allow the test device, specimen, and/or controls to reach room temperature (15-30°C) prior to testing.
- Remove the test from the foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
- Place the test device on a clean, flat surface.
- Shake the sample collection device several times.
- Holding the sample collection device upright, carefully break off the tip of collection device.
- Squeeze 3 drops (-90µL) of the sample solution in the sample well of the cassette and start the timer.
- Wait for the colored line(s) to appear. Read results in 5 minutes. Do not interpret the result after 5 minutes.
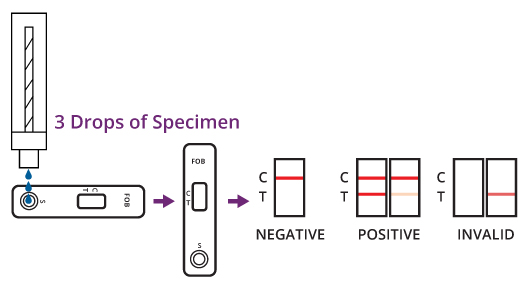
INTERPRETATION OF RESULTS
(Please refer to the illustration above)
Positive: Two lines appear. One colored line should be in the control line region (C) and another apparent colored line should be in the lest line region (T).
Negative: One colored line appears in the control line region (C). No line appears in the lest line region (T).
Invalid: Control line fails to appear. The test should be repeated using a new strip.If the problem persists, discontinue using the test kit immediately and contact your local distributor.
NOTE:
- The intensity of color in the test region (T) may vary depending on the concentration of analytes present in the specimen. Therefore, any shade of color in the test region should be considered positive. Note that this is a qualitative test only, and cannot determine the concentration of analytes in the specimen.
- Insufficient specimen volume, incorrect operating procedure or expired tests are the most likely reasons for control band failure.
QUALITY CONTROL
An internal procedural control is included in the test. A colored line appearing in the control line region (C) is an internal procedural control. It confirms sufficient specimen volume, adequate membrane wicking and correct procedural technique.
Control standards are not supplied with this kit; however it is recommended that positive and negative controls be tested as a good laboratory practice to confirm the test procedure and to verify proper test performance.
LIMITATIONS
- This test kit is to be used for the qualitative detection of human hemoglobin in fecal samples. A positive result suggests the presence of human hemoglobin in fecal samples. In addition lo intestinal bleeding the presence of blood in stools may have other causes such as hemorrhoids, blood in urine etc.
- Not all colorectal bleedings are due to precancerous or cancerous polyps. The information obtained by this lest should be used in conjunction with other clinical findings and testing methods, such as colonoscopy gathered by the physician
- Negative results do not exclude bleeding since some polyps and colorectal region cancers can bleed intermittently or not at all. Additionally, blood may not be uniformly distributed in fecal samples. Colorectal polyps al an early stage may not bleed.
- Urine and excessive dilution of sample with water from toilet bowl may cause erroneous test results. The use of a receptacle is recommended.
- Feces specimens should not collect during the menstrual period and not three day before or afterwards, at bleeding due to constipation, bleeding hemorrhoids, or at taking rectally administered medication. It could cause false positive results.
- This test may be less sensitive for detecting upper g.i. Bleeding because blood degrades as ii passes through the g.i. Track.
- The Fecal Occult Blood Rapid Test Cassette (Feces) is lo aid in diagnosis and is not intended to replace other diagnostic procedures such as G.I. fiberscope, endoscopy, colonoscopy, or X-ray analysis. Test results should not be deemed conclusive with respect to the presence or absence of gastrointestinal bleeding or pathology. A positive result should be followed up with additional diagnostic procedures to determine the exact cause and source for the occult blood in the feces.
PERFORMANCE CHARACTERISTICS
- Sensitivity:
Fecal Occult Blood Rapid Test Cassette (Feces) can detect the levels of human occult blood as low as 100ng/mL hemoglobin or 12µg hemoglobin/g feces.
- Prozone Effect:
It is observed that this FOB test can detect 2mg/ml hemoglobin.
- Specificity:
Fecal Occult Blood Rapid Test Cassette {Feces) is specific lo human hemoglobin. Specimen containing the following substances al the standard concentration was tested on both positive and negative controls and showed no effects on test results at standards concentration.