 |
 |
 |
 |
Laparoscopy Monopolar Electrode
Performance, Safety
With our cutting-edge technology and unwavering commitment to excellence, we bring you a range of electrodes designed to elevate your surgical experience. Whether you're seeking reliable hemostasis or precise tissue coagulation, trust EndoGuidance to deliver unparalleled performance and safety.
INTENDED USE
The EndoGuidance Laparoscopy Monopolar Electrode is provided with unipolar electrosurgical capacity. This device is used in a variety of laparoscopic procedures to coagulate tissue and does not contain latex.
SCOPE OF APPLICATION
This device is used for coagulation in minimally invasive surgery with high-frequency surgical equipment. However, it is not suitable for use in patients with severe cardiopulmonary insufficiency, blood coagulation disorders, acute diffuse peritonitis, or abdominal adhesions.
EMC (Electromagnetic Compatibility)
- The active medical device is suitable for use in the electromagnetic environment specified.
- RF emissions are very low and are not likely to cause interference in nearby electronic devices.
- This unit can be affected by portable and mobile RF communications equipment.
- Avoid using mobile phones or devices emitting electromagnetic fields near the unit as it may result in incorrect operation.
- Ensure proper installation and service according to provided EMC information.
- Do not use this machine adjacent to or stacked with other equipment. If necessary, verify normal operation in the intended configuration.
|
Guidance and manufacture’s declaration – electromagnetic emission
|
|
The active medical device is intended for use in the electromagnetic environment specified below. The customer of the user of the active medical device should assure that it is used in such an environment.
|
|
Emission test
|
Compliance
|
Electromagnetic environment – guidance
|
|
RF emissions CISPR 11
|
Group 2
|
The active medical device uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic
|
|
RF emission CISPR 11
|
Class A
|
The active medical device is suitable for use in all establishments, other than domestic and those directly connected to the public low-voltage power supply network that supplies buildings used for domestic purposes.
|
|
Harmonic emissions IEC 61000-3-2
|
Not Applicable
|
|
Voltage fluctuations/ flicker emissions IEC 61000-3-3
|
Not Applicable
|
- This product needs special precautions regarding EMC and needs to be installed and put into service according to the EMC information provided, and this unit can be affected by portable and mobile RF communications equipment.
- Do not use a mobile phone or other devices that emit electromagnetic fields, near the unit. This may result in incorrect operation of the unit.
- Caution: This unit has been thoroughly tested and inspected to assure proper performance and operation!
- Caution: this machine should not be used adjacent to or stacked with other equipment and that if adjacent or stacked use is necessary, this machine should be observed to verify normal operation in the configuration in which it will be used.
- Active Medical Devices include below models:
- EndoGuidance Laparoscopy Monopolar Electrodes: MDMPJ-33, MDMPL-33
CONTRAINDICATIONS
This device is not intended for contraceptive coagulation on fallopian tissue. It may be used to achieve hemostasis following transaction of the fallopian tube.
DIRECTIONS FOR USE
- Insert the Hook through the desired 5mm trocar sheath close to the tissue needing to be coagulated.
- The device is equipped with a standard 4mm male plug connector. To operate the unipolar electrosurgical feature, follow the instructions provided by the electrosurgical generator manufacturer.
- After completing the endoscopic procedure, dispose of this device following local regulations.
CAUTIONS/WARNINGS
- This device is for single-use only and cannot be reused or re-sterilized. Do not use if the package is opened or damaged.
- Only physicians with adequate training and knowledge of these procedures should perform endoscopic procedures. Consult medical literature regarding techniques, hazards, contraindications, and complications before performing procedures.
- Before Endoscopic instruments and accessories from different manufacturers are utilized together in a procedure, verify compatibility and ensure that electrical isolation of grounding is not compromised.
- A thorough understanding of techniques and principles involved in electrosurgical procedures is necessary to avoid burn or shock hazard to patient and/or operator.
- This instrument should be used with U.L recognized unipolar electrosurgical generators, with capability of being activated with a foot control switch.
- Keep the voltage power as low as possible to achieve the desired end effect. Refer to use instructions provided by the electrosurgical generator to ensure that all safety precautions are followed.
- Using a metal trocar sheath in conjunction with a plastic site stability device creates a potential hazard when using electrosurgical instruments.
- Federal (U.S.A) law restricts this device to sale, distribution, and use by, or on the order of a physician.
- The highest voltage of the high frequency generator connected with the product is not more than 2500V.
- Suggested parameter adjustment:
- 10.1 In the single cut mode, the generator is 50-100W, and the maximum is not more than 150W.
- 10.2 In the single setting mode, the generator is 30-60W, and the maximum is not more than 100W.
- 10.3 In the mixing mode, the generator is 40-80W, and the maximum is not more than 120W.
- If it is found that the shell of the product or the insulating cortex is damaged or abnormal, do not use it.
The EndoGuidance Laparoscopy Electrode Instruments are crafted to enhance your surgical procedures, providing unmatched precision and control in minimally invasive surgery. Featuring an auto-shield mechanism for effortless penetration, they reduce tissue trauma, fostering swift recovery.
Monopolar Instruments Tips
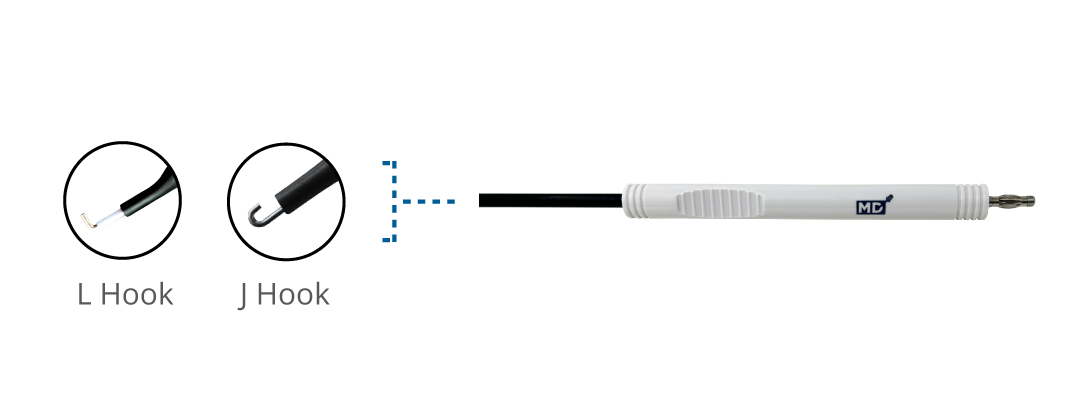
Laparoscopic Monopolar Electrode
|
|
|
|
|
|
|
|
|
|
|
Products Description
|
Product Code
|
Size
|
Image
|
|
Monopolar
L Hook (L Shaped)
|
MDMPL-33
|
5mm x 330mm
|

|
|
Monopolar
J Hook (J Shaped)
|
MDMPJ-33
|
5mm x 330mm
|

|
|
|
|
|
|
|
|
|
|
|
Usage Guidelines:
- Training: Ensure all users are trained in proper instrument handling and safety protocols.
- Inspection: Check instruments for damage before each use; do not use if damaged.
- Sterilization: Follow manufacturer's sterilization instructions strictly.
- Electrosurgery Safety: Use caution with electrosurgical units to prevent accidental harm.
- Gentle Handling: Handle tissue delicately to avoid damage.
- Monitoring: Continuously monitor tissue effects during surgery; adjust settings as needed.
- Post-Op Care: Provide appropriate care to minimize complications and promote healing.
- Documentation: Maintain detailed records of usage, maintenance, and any complications.
- Quality Assurance: Implement measures to ensure instrument safety and effectiveness.
- Regulatory Compliance: Adhere to all relevant regulations and standards.
Precautions:
- Electrical Safety: Exercise caution to prevent electrical burns or shocks. Ensure proper grounding and insulation of equipment.
- Tissue Damage: Be mindful of the potential for unintended tissue damage due to the electrical current. Use appropriate power settings and techniques to minimize tissue trauma.
- Patient Positioning: Ensure proper patient positioning to minimize the risk of injury from electrical currents traveling through the body.
- Instrument Handling: Handle monopolar instruments with care to avoid accidental contact with nearby conductive materials or fluids.
- Avoiding Flammable Materials: Keep flammable materials away from the surgical field to prevent the risk of ignition from electrical sparks.
- Training and Competency: Ensure that all surgical staff using monopolar instruments are adequately trained and competent in their use to mitigate the risk of errors or accidents.
- Monitoring and Supervision: Continuously monitor the surgical site and instrument performance during procedures, and have a supervising surgeon available to intervene if necessary.
- Emergency Preparedness: Be prepared to respond promptly to any adverse events or complications that may arise during surgery, including unintended tissue damage or electrical burns.
- Patient Selection: Consider the patient's medical history and condition when determining the suitability of using monopolar instruments, particularly in cases where there may be an increased risk of complications.
- Follow Manufacturer Guidelines: Adhere to the manufacturer's instructions for the proper use and maintenance of monopolar instruments to ensure optimal performance and safety.
By adhering to these precautions, healthcare providers can minimize the risks associated with using monopolar instruments and ensure the safety of both patients and surgical staff.
Note
This product information is provided for reference purposes. Please consult the detailed product documentation and adhere to your institution's guidelines for usage.

|
Sterilized by Ethylene Oxide
|
You can download the MD Monopolar Electrode product insert

You can download this product flyer

|
|
 |
 |
 |
 |

|